BD MAX™ Vaginal Panel
Elevate the standard of care for Women’s Health
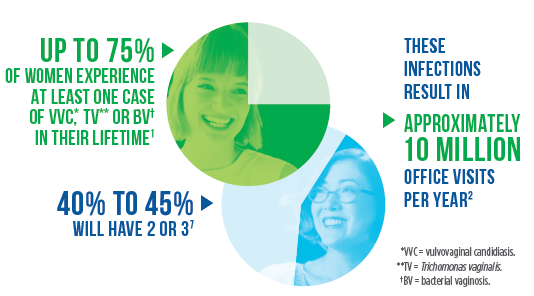
 Vaginal infections are among the most common reasons for which women in the United States seek medical care–resulting in approximately 10 million visits to physician offices annually.1,2,3,4 Traditional diagnostic techniques tend to be subjective with variable sensitivity and specificity.2,5,6 This potentially leads to continued symptoms, repeat visits, inappropriate treatment, poor antimicrobial stewardship and unnecessary associated healthcare system costs.1,7,8
Vaginal infections are among the most common reasons for which women in the United States seek medical care–resulting in approximately 10 million visits to physician offices annually.1,2,3,4 Traditional diagnostic techniques tend to be subjective with variable sensitivity and specificity.2,5,6 This potentially leads to continued symptoms, repeat visits, inappropriate treatment, poor antimicrobial stewardship and unnecessary associated healthcare system costs.1,7,8
Introducing the BD MAX™ Vaginal Panel
The BD MAX Vaginal Panel is the first FDA market-authorized, microbiome-based, polymerase chain reaction (PCR) assay that directly detects the 3 most common infectious causes of vaginitis: Bacterial vaginosis, vulvovaginal candidiasis and trichomoniasis.9
With the BD MAX Vaginal Panel, you can maximize efficiency with 1 collection, 1 test for the 3 most common infectious causes of vaginitis.9 This assay also supports antimicrobial resistance initiatives by reporting Candida krusei and C. glabrata.
- Download the BD MAX™ Vaginal Panel Info Sheet >>
- Download the BD MAX™ Vaginal Specimen Self-Collection Collection Chart>>
- Watch the BD MAX™ Vaginal Panel Specimen Self-Collection Video
- Download the BD MAX™ Vaginal Panel Specimen Collection and Transfer Procedure Collection Chart>>
- Watch the BD MAX™ Vaginal Panel Specimen Collection and Transfer Procedure Video
- Read how patients may receive appropriate treatment sooner while avoiding the expense and burden of unnecessary care with BD MAX™ Vaginal Panel. White Paper>>
- Browse the Resource Center to access scientific publications and more
Visit bd.com for detailed information on the BD MAX™ Vaginal Panel (Cat No. 443712).
Reference
- Hainer BL et al. Vaginitis. Am Fam Physician. 2011;83(7):807-815.
- Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165(4 Pt 2):1168-1176.
- Sherrard J et al. European (IUSTI/WHO) guideline on the management of vaginal discharge. Int J STD AIDS. 2011;22(8):421-429.
- Workowski KA et al; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Gutman RE et al. Evaluation of clinical methods for diagnosing bacterial vaginosis. Obstet Gynecol. 2005;105(3):551-556.
- Menard JP et al. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis. 2008;47(1):33-43.
- Carr PL et al. “Shotgun” versus sequential testing. Cost-e ectiveness of diagnostic strategies for vaginitis. JGIM. 2005;793-799.
- Powell K. Vaginal thrush: quality of life and treatments. Br J Nurs. 2010;19:1107-1111.
- Package Insert/Clinical Trial Data.
 Vaginal infections are among the most common reasons for which women in the United States seek medical care–resulting in approximately 10 million visits to physician offices annually.1,2,3,4 Traditional diagnostic techniques tend to be subjective with variable sensitivity and specificity.2,5,6 This potentially leads to continued symptoms, repeat visits, inappropriate treatment, poor antimicrobial stewardship and unnecessary associated healthcare system costs.1,7,8
Vaginal infections are among the most common reasons for which women in the United States seek medical care–resulting in approximately 10 million visits to physician offices annually.1,2,3,4 Traditional diagnostic techniques tend to be subjective with variable sensitivity and specificity.2,5,6 This potentially leads to continued symptoms, repeat visits, inappropriate treatment, poor antimicrobial stewardship and unnecessary associated healthcare system costs.1,7,8